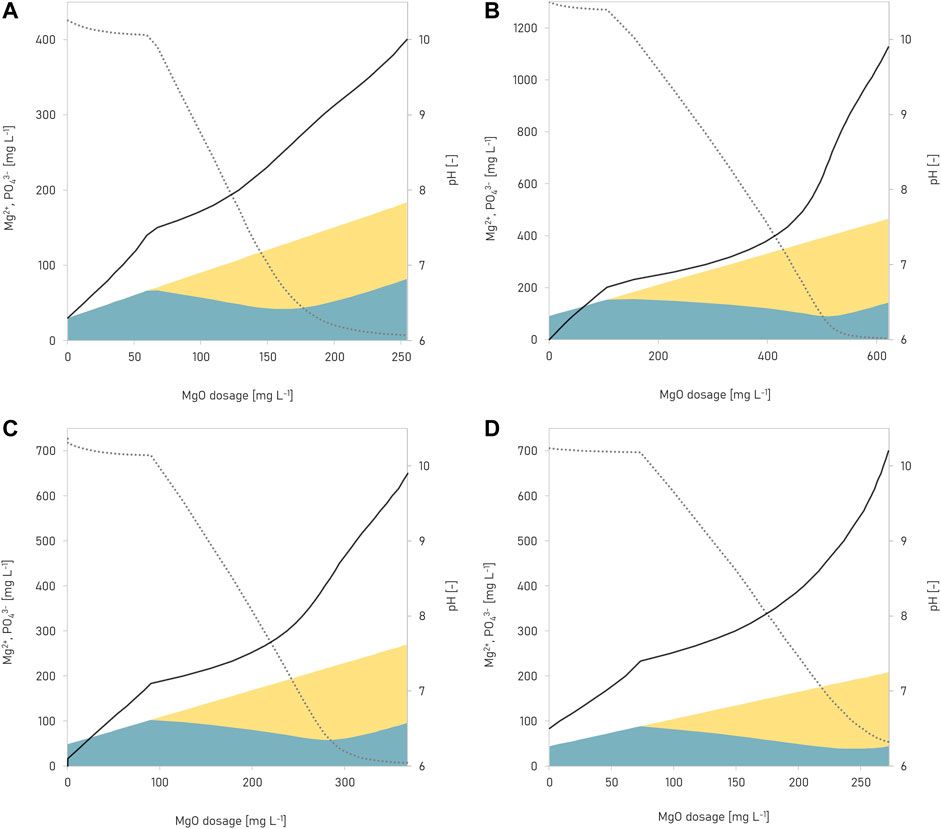
Here’s a new paper we published in Frontiers in Environmental Science: https://www.frontiersin.org/articles/10.3389/fenvs.2022.889119/full
In the study, we systematically analysed the kinetics and thermodynamics of how magnesium hydroxide dissolves in different types of human urine (fresh urine and fresh urine concentrated by evaporation). We showed that Mg hydroxide has a unique dissolution behaviour which is unlike that of other alkaline earth hydroxides, especially when water is removed from urine. We detail conditions and design criteria for alkalising and for dehydrating urine, which is useful when developing source-separating sanitation systems.
A cool aspect of the study was that we found a smart way to simulate the kinetics of how fast Mg hydroxide dissolves, how fast various precipitates form (e.g. struvite, apatite), and how fast urine is alkalised and saturated. We did this by matching the experimentally measured pH of urine with its thermodynamically simulated pH. This approach could be applied to any system as long as the parameter we measure is something we can also simulate thermodynamically.